potassium ion electron configuration|how many electrons does potassium have : Pilipinas In order to write the Potassium electron configuration we first need to know the number of electrons for the K atom (there are 19 electrons). When we write the configuration we'll . Yuko, who remarried half a year ago, was worried about her relationship with her adolescent son-in-law. Not knowing such a thing, her husband was dissatisfied with the lack of night life and planned to obtain a super powerful aphrodisiac and give Yuko a drink. However, Yuko, who is sick in the afternoon without her husband, may be mistaken for a .Jones vs. Gustafsson at UFC 165 on Tapology. View Jones vs. Gustafsson fight video, highlights, news, Twitter updates, and fight results.
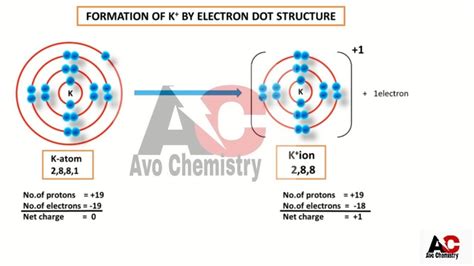
potassium ion electron configuration,In this video we will write the electron configuration for K+, the Potassium ion. We’ll also look at why Potassium forms a 1+ ion and how the electron configuration for K+ is the same as the.In order to write the Potassium electron configuration we first need to know the number of electrons for the K atom (there are 19 electrons). When we write the configuration we'll . Well, potassium has Z=19... And Z specifies the nuclear charge. For a neutral element there must be 19 electrons, 19 fundamental particles of negative .The electron configuration of potassium is [Ar] 4s1. The configuration of potassium is carried out according to the rules derived from Klechkovskaya, namely: Ar: 4s1, taking .Thus, potassium has an electron configuration of [Ar]4s 1. Hence, potassium corresponds to Li and Na in its valence shell configuration. The next electron is added to complete the 4s subshell and calcium .
potassium ion electron configuration A step-by-step description of how to write the electron configuration for Potassium (K). In order to write the K electron configuration we first need to kno.potassium ion electron configuration how many electrons does potassium have A step-by-step description of how to write the electron configuration for Potassium (K). In order to write the K electron configuration we first need to kno.
Electron Configuration for Potassium Ion. You can see the electron configuration for K Ion in the picture below; How Many Valence Electrons Are in K? The potassium Number of Valence .Atoms gain or lose electrons to form ions with particularly stable electron configurations. The charges of cations formed by the representative metals may be determined readily .
Thus, potassium has an electron configuration of [Ar]4s 1. Hence, potassium corresponds to Li and Na in its valence shell configuration. The next electron is added .Write the electron configurations of these cations. Solution. First, write the electron configuration for the neutral atoms: Zn: [Ar]3 d10 4 s2. Cr: [Ar]3 d5 4 s1. Next, remove electrons from the highest energy orbital. For the transition metals, electrons are removed from the s orbital first and then from the d orbital.Answer and Explanation: 1. Become a Study.com member to unlock this answer! Create your account. View this answer. Recall: The atomic number of potassium ion is: Z=19. The expression for the electron configuration for a potassium ion is, {eq}1s^22s^22p^63s^23p. See full answer below.⬆️⬆️⬆️ The electron configuration of potassium is 4s1. The configuration of potassium is carried out according to the rules derived from Klechkovskaya, . thus creating a mono-positive ion. Potassium has a very low ionization energy and very low electron affinity. It has a melting point of 336.53 degrees Kelvin and a boiling point .

For instance, the ground state electronic configuration of calcium (Z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. The calcium ion (Ca 2+), however, has two electrons less. Hence, the electron configuration for Ca 2+ is 1s 2 2s 2 2p 6 3s 2 3p 6. Since we need to take away two electrons, we first remove electrons from the outermost shell (n=4).
Thus, potassium has an electron configuration of [Ar]4s 1. Hence, potassium corresponds to Li and Na in its valence shell configuration. . Electron Configurations of Ions. We have seen that ions are formed when atoms gain or lose electrons. A cation (positively charged ion) forms when one or more electrons are removed from a parent . Thus, potassium has an electron configuration of [Ar]4s 1. Hence, potassium corresponds to Li and Na in its valence shell configuration. . Electron Configurations of Ions. We have seen that ions are formed when atoms gain or lose electrons. A cation (positively charged ion) forms when one or more electrons are .This electron configuration is written as 1 s2 2 s1. The next element is beryllium, with Z = 4 and four electrons. We fill both the 1 s and 2 s orbitals to achieve a 1 s2 2 s2 electron configuration: When we reach boron, with Z = 5 and five electrons, we must place the fifth electron in one of the 2 p orbitals. Electron configuration chart of all Elements is mentioned in the table below.The Shorthand electron configuration (or Noble gas configuration) as well as Full . Electron configuration of Potassium (K) [Ar] 4s 1: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1: 2, 8, 8, 1: 20: Electron configuration of Calcium (Ca) [Ar] 4s 2: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2: In the case of the 19th element, the color is pale lavender. Like sodium ions, the presence of potassium ions in the body is essential for the correct function of many cells. Table \(\PageIndex{1}\): Basic Chemical and Physical Properties; Atomic Number: 19: . Electron Configuration: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1: Notable Reactions with .
Il existe 25 isotopes connus du potassium, dont trois se produisent naturellement : 39K (93,3 %), 40K (0,0117 %) et 41K (6,7 %). Le potassium 39 est composé de 19 protons, 20 neutrons et 19 électrons. Potassium - Protons - Neutrons - Électrons - .
how many electrons does potassium haveWrite the electron configuration for a potassium ion. Solution. First, write the electron configuration for the neutral atom: K: [Ar]4s 1; Next, remove electrons from the highest energy orbital. Potassium is a member of group 1, so it should have a charge of 1+, and thus loses one electron from its s orbital. This gives the following electron .
Add an electron to the anion electron configuration. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. For Cl −, it will be 1s²2s²2p⁶3s²3p⁶. Remove the outermost electrons in the cation, e.g. electron configuration for Mg 2+ will be 1s²2s²2p⁶.The full electron configuration of potassium is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1; Shorthand Electron Configurations. Using potassium as an example again: . To form a magnesium ion, it loses its two outer electrons so the electronic configuration for the ion is: 1s 2 .

Thus, potassium has an electron configuration of [Ar]4s 1. Hence, potassium corresponds to Li and Na in its valence shell configuration. . Electron Configurations of Ions. Ions are formed when atoms gain or lose electrons. A cation (positively charged ion) forms when one or more electrons are removed from a parent . The ground state electron configuration of potassium is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 1.. Excited state of Potassium electron configuration. The excited state of the potassium electronic configuration is present as 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 0.. Ground state Potassium orbital diagram. Potassium’s orbital diagram in its ground .
If we lose two electrons, we have a net deposited two charge. We form the calcium to ion. The two electrons that we would lose to form the calcium two plus ion are these. These two electrons right here in the 4s orbital. The electron configuration for calcium two plus would be the same as the electron configuration for the noble gas argon here.Potassium. 19. 39.098. Glossary. GroupA vertical column in the periodic table. Members of a group typically have similar properties and electron configurations in their outer shell. PeriodA horizontal row in the periodic table. The atomic number of each element increases by one, reading from left to right.Potassium (K) is a chemical element with the atomic number 19. Its electronic configuration is [2, 8, 8, 1]. This means that it has 19 electrons distributed in its atomic orbitals. To understand potassium’s electron configuration, we need to look at its atomic structure. Potassium has a total of 19 electrons.
potassium ion electron configuration|how many electrons does potassium have
PH0 · writing electron configurations for ions
PH1 · what is the configuration for k
PH2 · shorthand electron configuration for potassium
PH3 · k+ ion electron configuration
PH4 · how many valence electrons potassium
PH5 · how many electrons does potassium have
PH6 · electron configuration of v3+ ion
PH7 · electron configuration chart
PH8 · Iba pa